FDA Approves Angiotensin-II for Septic Shock
pulmccm.org
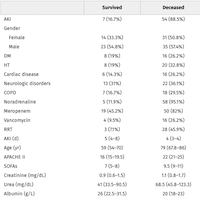
The FDA approved angiotensin-II (Giapreza) as a new intravenous vasopressor for septic shock and other forms of distributive shock. The first new FDA-approved vasopressor in decades, angiotensin-II could significantly change the management of severe septic shock. FDA based its expedited approval (under priority review) on the ATHOS-3 trial enrolling 321 patients with shock refractory to catecholamines like norepinephrine or epinephrine.