Clinical Trials Design Evaluating Sedation in Critically Ill Adults Requiring Mechanical Ventilation
journals.lww.com
These recommendations are intended to assist researchers in the design, conduct, selection of endpoints, and reporting of clinical trials involving sedative medications and/or sedation protocols for adult ICU patients who require mechanical ventilation.
These recommendations should be viewed as a starting point to improve clinical trials and help reduce methodological heterogeneity in future clinical trials.
The final recommendations were iteratively refined based on the survey results, participants’ reactions to those results, summaries written by panel moderators, and a review of the meeting transcripts made from audio recordings.
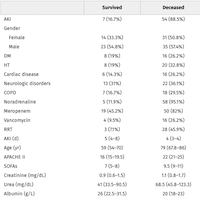
36 participants from academia, industry, and the Food and Drug Administration with expertise in relevant content areas, including two former ICU patients attended the in-person meeting, and the majority completed an online follow-up survey and participated in the modified Delphi process.