Temporal development and neutralising potential of antibodies against SARS-CoV-2 in hospitalised COVID-19 patients
journals.plos.org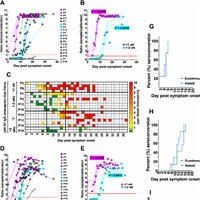
Antibody responses are important in the control of viral respiratory infection in the human host. What is not clear for SARS-CoV-2 is how rapidly this response occurs, or when antibodies with protective capability evolve. Hence, defining the events of SARS-CoV-2 seroconversion and the time frame for the development of antibodies with protective potential may help to explain the different clinical presentations of COVID-19.
Furthermore, accurate descriptions of seroconversion are needed to inform the best use of serological assays for diagnostic testing and serosurveillance studies.
Here, we describe the humoral responses in a cohort of hospitalised COVID-19 patients (n = 19) shortly following the onset of symptoms. Commercial and ‘in-house’ serological assays were used to measure IgG antibodies against different SARS-CoV-2 structural antigens–Spike (S) S1 sub-unit and Nucleocapsid protein (NP)–and to assess the potential for virus neutralisation mediated specifically by inhibition of binding between the viral attachment protein (S protein) and cognate receptor (ACE-2).
Antibody response kinetics varied amongst the cohort, with patients seroconverting within 1 week, between 1–2 weeks, or after 2 weeks, following symptom onset.
Anti-NP IgG responses were generally detected earlier, but reached maximum levels slower, than anti-S1 IgG responses.
The earliest IgG antibodies produced by all patients included those that recognised the S protein receptor-binding domain (RBD) and were capable of inhibiting binding to ACE-2.
These data revealed events and patterns of SARS-CoV-2 seroconversion that may be important predictors of the outcome of infection and guide the delivery of clinical services in the COVID-19 response.