Use of Bivalirudin for Anticoagulation in Pediatric ECMO
journals.sagepub.com
This study describes the use of bivalirudin in children on extracorporeal membrane oxygenation (ECMO). Pediatric patients receiving bivalirudin were compared to patients receiving heparin as the anticoagulant on ECMO.
Data was collected for children under 18 years of age supported by ECMO from January 2016 to December 2019.
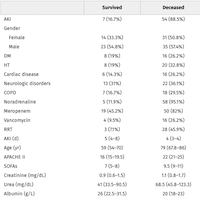
Data collected included demographics, diagnosis, ECMO indication, type, and duration, indication for bivalirudin use, dose range, activated partial thromboplastin time (aPTT) levels, minor and major bleeding, hemolysis, and mortality. Forty pediatric patients received ECMO; eight received bivalirudin primarily for anticoagulation.
The median age was 4 months (IQR 0.5, 92) in the heparin cohort, 0.6 months (IQR 0.0, 80.0) in the primary bivalirudin cohort.
The indication for ECMO was respiratory in 5 patients (18%) in the heparin group versus 6 (75%) in the primary bivalirudin group, cardiac in 18 (67%) in heparin versus 1 (12.5%) in primary bivalirudin, and extracorporeal-cardiopulmonary resuscitation (E-CPR) in 4 (15%) in heparin versus 1 (12.5%) in primary bivalirudin. Bivalirudin was the initial anticoagulant for eight patients (66.6%) while three (25%) were switched due to concern for heparin-induced thrombocytopenia (HIT) and one (8%) for heparin resistance.
The median time to achieve therapeutic aPTT was 14.5 hours compared to 12 hours in the heparin group.